IMPORTANT SAFETY INFORMATION
SEVERE HYPOCALCEMIA IN PATIENTS WITH ADVANCED KIDNEY DISEASE:
Patients with advanced chronic kidney disease are at greater risk of severe hypocalcemia following Prolia administration. Severe hypocalcemia resulting in hospitalization, life-threatening events and fatal cases have been reported. The presence of chronic kidney disease-mineral bone disorder (CKD-MBD) markedly increases the risk of hypocalcemia. Prior to initiating Prolia in patients with advanced chronic kidney disease, evaluate for the presence of CKD-MBD. Treatment with Prolia in these patients should be supervised by a healthcare provider with expertise in the diagnosis and management of CKD-MBD.
Contraindications: Prolia® is contraindicated in patients with
hypocalcemia. Pre-existing hypocalcemia must be corrected prior to initiating
Prolia®. Prolia® is contraindicated in women who are pregnant and may
cause fetal harm. In women of reproductive potential, pregnancy testing should be performed
prior to initiating treatment with Prolia®. Prolia® is contraindicated
in patients with a history of systemic hypersensitivity to any component of the product.
Reactions have included anaphylaxis, facial swelling and urticaria.
Severe Hypocalcemia and Mineral Metabolism Changes: Prolia can cause severe hypocalcemia and fatal cases have been reported. Pre existing hypocalcemia must be corrected prior to initiating therapy with Prolia. Adequately supplement all patients with calcium and vitamin D.
In patients without advanced chronic kidney disease who are predisposed to hypocalcemia and disturbances of mineral metabolism (e.g. treatment with other calcium lowering drugs), assess serum calcium and mineral levels (phosphorus and magnesium) 10 to14 days after Prolia injection.
Same Active Ingredient: Prolia® contains the same active ingredient
(denosumab) found in XGEVA®. Patients receiving Prolia® should not receive
XGEVA®.
Hypersensitivity: Clinically significant hypersensitivity including anaphylaxis has been reported with Prolia®. Symptoms have
included hypotension, dyspnea, throat tightness, facial and upper airway edema, pruritus and urticaria. If an anaphylactic or other
clinically significant allergic reaction occurs, initiate appropriate therapy and discontinue further use of Prolia®.
Osteonecrosis of the Jaw (ONJ): ONJ, which can occur spontaneously, is generally
associated with tooth extraction and/or local infection with delayed healing, and has been
reported in patients receiving Prolia®. An oral exam should be performed by the
prescriber prior to initiation of Prolia®. A dental examination with appropriate
preventive dentistry is recommended prior to treatment in patients with risk factors for ONJ
such as invasive dental procedures, diagnosis of cancer, concomitant therapies (e.g.,
chemotherapy, corticosteroids, angiogenesis inhibitors), poor oral hygiene, and co-morbid
disorders. Good oral hygiene practices should be maintained during treatment with
Prolia®. The risk of ONJ may increase with duration of exposure to
Prolia®.
For patients requiring invasive dental procedures, clinical judgment should guide the
management plan of each patient. Patients who are suspected of having or who develop ONJ
should receive care by a dentist or an oral surgeon. Extensive dental surgery to treat ONJ
may exacerbate the condition. Discontinuation of Prolia® should be considered
based on individual benefit-risk assessment.
Atypical Femoral Fractures: Atypical low-energy, or low trauma fractures of the shaft
have been reported in patients receiving Prolia®. Causality has not been
established as these fractures also occur in osteoporotic patients who have not been treated
with antiresorptive agents.
During Prolia® treatment, patients should be advised to report new or unusual
thigh, hip, or groin pain. Any patient who presents with thigh or groin pain should be
evaluated to rule out an incomplete femur fracture. Interruption of Prolia®
therapy should be considered, pending a risk/benefit assessment, on an individual basis.
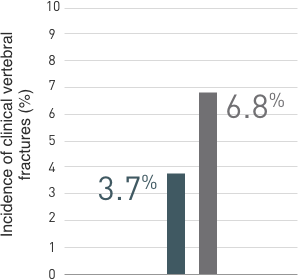
Multiple Vertebral Fractures (MVF) Following Discontinuation of Prolia®
Treatment: Following discontinuation of Prolia® treatment, fracture risk increases, including the risk of multiple vertebral fractures. New vertebral fractures occurred as early as 7 months (on average 19 months) after the last dose of Prolia®. Prior vertebral fracture was a predictor of multiple vertebral fractures after Prolia® discontinuation. Evaluate an individual’s benefit/risk before initiating treatment with Prolia®. If Prolia® treatment is discontinued, consider transitioning to an alternative antiresorptive therapy.
Serious Infections: In a clinical trial (N=7808), serious infections leading to hospitalization were reported more frequently in the Prolia® group than in the placebo group. Serious skin infections, as well as infections of the abdomen, urinary tract and ear were more frequent in patients treated with
Prolia®.
Endocarditis was also reported more frequently in Prolia®-treated patients. The
incidence of opportunistic infections and the overall incidence of infections were similar
between the treatment groups. Advise patients to seek prompt medical attention if they
develop signs or symptoms of severe infection, including cellulitis.
Patients on concomitant immunosuppressant agents or with impaired immune systems may be at
increased risk for serious infections. In patients who develop serious infections while on
Prolia®, prescribers should assess the need for continued Prolia®
therapy.
Dermatologic Adverse Reactions: In the same clinical trial in women with
postmenopausal osteoporosis, epidermal and dermal adverse events such as dermatitis, eczema
and rashes occurred at a significantly higher rate with Prolia® compared to
placebo. Most of these events were not specific to the injection site. Consider
discontinuing Prolia® if severe symptoms develop.
Musculoskeletal Pain: Severe and occasionally incapacitating bone, joint, and/or
muscle pain has been reported in patients taking Prolia®. Consider discontinuing
use if severe symptoms develop.
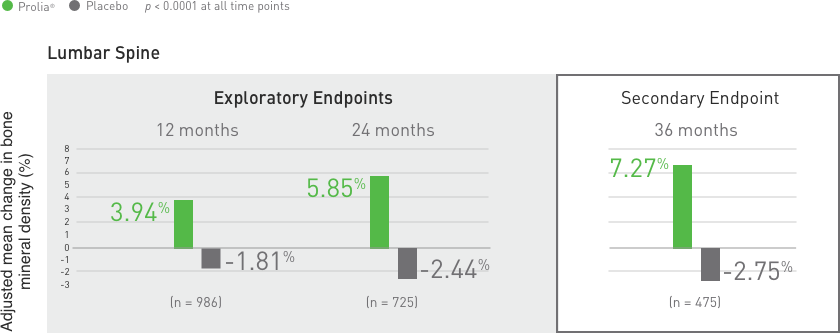
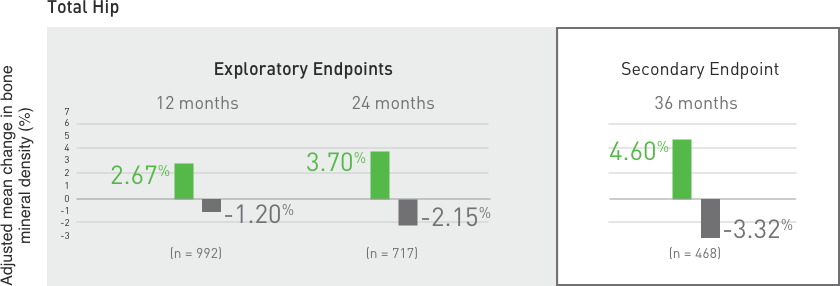
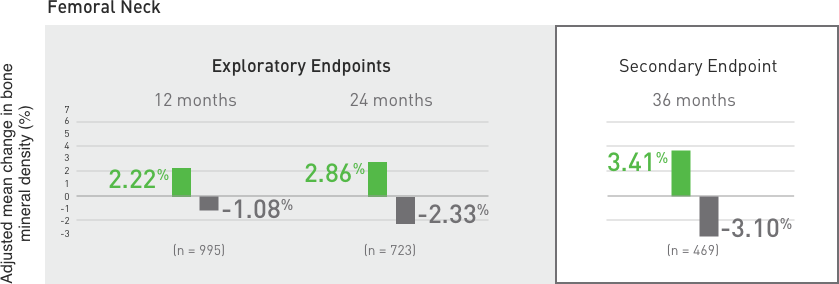
Suppression of Bone Turnover: Prolia® resulted in significant suppression of bone remodeling as evidenced by markers of bone turnover and bone histomorphometry. The significance of these
findings and the effect of long-term treatment are unknown. Monitor patients for consequences, including ONJ, atypical fractures, and delayed fracture healing.
Adverse Reactions: The most common adverse reactions (>5% and more common than
placebo) in women with postmenopausal osteoporosis are back pain, pain in extremity,
musculoskeletal pain, hypercholesterolemia, and cystitis. The most common adverse reactions
(>5% and more common than placebo) in men with osteoporosis are back pain, arthralgia,
and nasopharyngitis. Pancreatitis has been reported with Prolia®.
In women with postmenopausal osteoporosis, the overall incidence of new malignancies was 4.3%
in the placebo group and 4.8% in the Prolia® group. In men with osteoporosis, new
malignancies were reported in no patients in the placebo group and 4 (3.3%) patients in the
Prolia® group. A causal relationship to drug exposure has not been established.
The most common adverse reactions (>3% and more common than active-control group) in
patients with glucocorticoid-induced osteoporosis are back pain, hypertension, bronchitis,
and headache.
The most common (per patient incidence 10%) adverse reactions reported with
Prolia® in patients with bone loss receiving ADT for prostate cancer or adjuvant
AI therapy for breast cancer are arthralgia and back pain. Pain in extremity and
musculoskeletal pain have also been reported in clinical trials. Additionally, in
Prolia®-treated men with nonmetastatic prostate cancer receiving ADT, a greater
incidence of cataracts was observed.
Denosumab is a human monoclonal antibody. As with all therapeutic proteins, there is
potential for immunogenicity.
Please see Prolia®
full
Prescribing Information, including Medication Guide.